Research
Recent Research Highlights
Development and application of an agent-based model for the simulation of the extravasation process of circulating tumor cells
Kay. M. Schneider1, Klaudia Giehl2, Stephan A. Baeurle1
1 Department of Chemistry and Biology, Universität Siegen, Siegen, Germany
2 Signal Transduction of Cellular Motility, Internal Medicine IV, Justus-Liebig University Giessen, Giessen, Germany
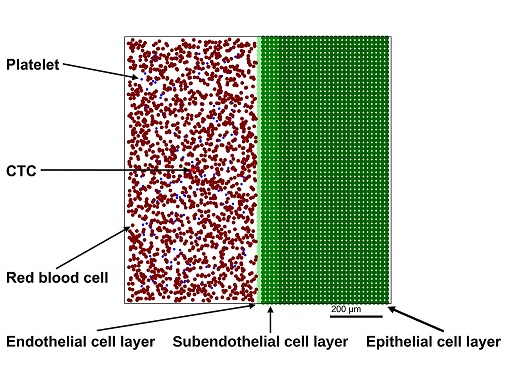
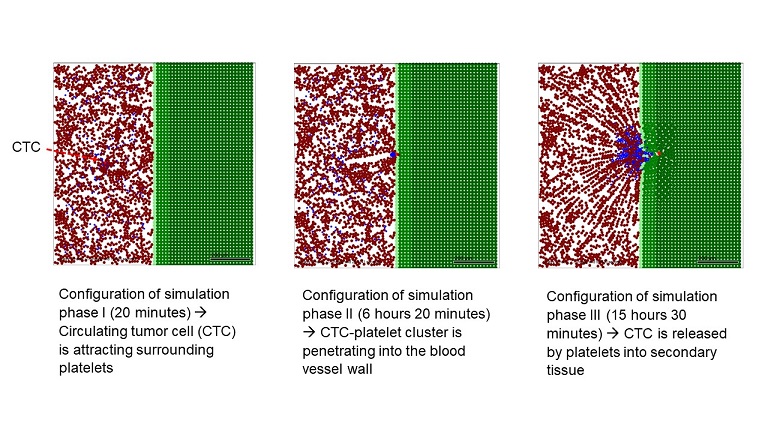
Abstract:
The primary cause for cancer-related death is metastasis, and although this phenomenon is the hallmark of cancer, it remains poorly understood. Since studies on the underlying mechanisms are still demanding by experimental means prognostic tools based on computer models can be of great value, not only for elucidating metastasis formation but also for assessing the prospective benefits as well as risks of a therapy for patients with advanced cancer. Here, we present an agent-based model (ABM), describing the complete process of platelet-assisted extravasation of circulating tumor cells (CTCs) from the chemoattraction of blood platelets by the CTCs up to the embedding of the CTCs in the epithelial tissue by computational means. From the simulation results, we conclude that the ABM produces results in consistency with experimental observations, which opens new perspectives for the development of computer models for predicting the efficacity of drug-based tumor therapies and assisting precision medicine approaches.
Publication:
K. M. Schneider, K. Giehl and S. A. Baeurle, Development and application of an agent-based model for the simulation of the extravasation process of circulating tumor cells, Int. J. Numer. Meth. Biomed. Engng., e3679 (2023); doi: 10.1002/cnm.3679
Study of protease-mediated process initiating viral infection and cell-cell viral spreading of SARS-CoV-2
Thanawat Thaingtamtanha1, Stephan A. Baeurle1
1 Department of Chemistry and Biology, Universität Siegen, Germany
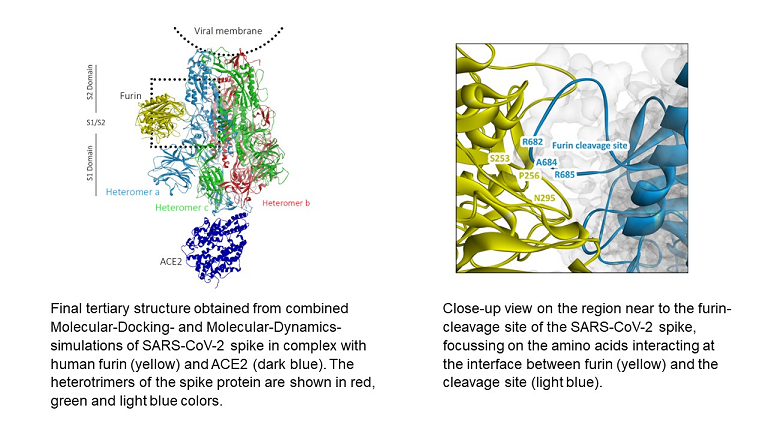
Abstract:
Viral-cell entry and cell-cell viral spreading processes of SARS-CoV-2 are subjected to fast evolutionary optimization because of its worldwide spreading, requiring the need for new drug developments. However, this task is still challenging, because a detailed understanding of the underlying molecular processes, mediated by the key cellular proteases TMPRSS2 and furin, is still lacking. Here, we show by large-scale atomistic calculations that binding of the ACE2 cell receptor at one of the heteromers of the SARS-CoV-2 leads to a release of its furin cleavage site (S1/S2), enabling an enhanced furin binding, and that this latter process promotes the binding of TMPRSS2 through the release of the TMPRSS2 cleavage site (S2') out of the ACE2-binding heteromer. Moreover, we find that, after proteolytic cleavage, improved furin binding causes that parts of the S2 subunit dissociate from the complex, suggesting that furin promotes the fusion of the S2 subunit with the cell membrane before transfer of the viral RNA.
Publications:
T. Thaingtamtanha and S. A. Baeurle, Study of protease-mediated processes initiating viral infection and cell-cell viral spreading of SARS-CoV-2, J. Mol. Model. 28, 224 (2022); doi: 10.1007/s00894-022-05206-8
