Current Projects
Construction of a Molecular Crane Based on the Flavoprotein Dodecin
Funded by the European Research Council, ERC
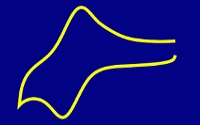
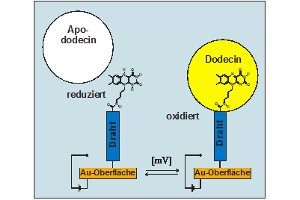
The flavoprotein dodecin from the halophilic organism Halobacterium salinarum binds not only native but also artificial flavins with high affinities in their oxidized state. Reduction of the flavins induces the dissociation of the holocomplex into apododecin and free flavin. Based on these unique binding characteristics, a molecular crane shall be developed that is able to pick up and to release molecular objects through a switch of the electric potential. For this purpose, a single flavin has to be linked to the conductive tip of an atomic force microscope via a molecular wire-like subunit (flavin molecular wire – AFM tip/electrode, see below). On the basis of such an electrochemically switchable molecular crane, it will be possible to bind and release single molecules of dodecin apoprotein or even larger molecular assemblies attached to apododecin serving as molecular junction.
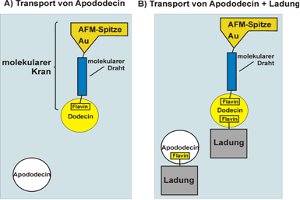
While the construction of a molecular crane for the transport of single molecules is the main goal, the successful realization of this project fundamentally depends on the synthesis and characterization of molecular wire-like subunits, which can be used to attach redox-active proteins to surfaces in an electrochemically switchable state. Thus, functionalized single-walled carbon nanotubes or organic ?-electron systems will be examined with respect to their ability to serve as molecular wire. Our strategy is to develop electrochemically addressable flavin modified surfaces, and to stepwise miniaturize successful assemblies down to the single molecule level.
Surface modification protocols have to be developed and modified surfaces will be investigated by a combination of atomic force microscopy, surface plasmon resonance spectroscopy, ellipsometry, quartz crystal microbalance (QCM) measurements and electrochemical methods. The results of these studies will be of general interest for the construction of molecular switches, devices, and transport systems, and for the development of amperometric biosensors and biofuel cells.
Investigation of Elektron Transfer Processes through DNA and Development of an electrochemical DNA-Sensor
Funded by the country NRW
In our previous work, double-stranded DNA (ds-DNA) of 20 base pairs was used in order to tether flavins to gold surfaces in an electrochemically active configuration. It turned out that the conductivity of DNA is too low to serve as molecular wire (M. Grininger and G. Nöll, S. Trawöger, E.-K. Sinner, and D. Oesterhelt; Biointerphases, 2008, 3, 51-58). The applied DNA base sequence, as well as the tethers to bind flavin and the thiol (protected as disulfide), which is required for adsorption to gold, are shown below.

Nevertheless it is known that excess electron transfer (ET) through DNA is possible over large distances. Therefore we are evaluating how ET through DNA monolayers can be optimized, and if there is a possibility to develop an electrochemical DNA sensor in order to trace single base mismatches based on ET along the DNA base stacks. During these experiments a flavin, which is covalently linked to DNA, will serve as redox-active moiety. Of strong interest is the role of the tethers between DNA and flavin, as well as DNA and the gold surface, with respect to ET. In particular, the length and the point of attachment of the thiol bearing tether to DNA shall be varied, i.e. tethers of different length can be attached to the 3’ or 5’ end of DNA. Furthermore, different surface modification procedures shall be examined in order to optimize the architecture of the single-stranded DNA (ss-DNA) modified electrode surface with respect to a sensor application. The individual surface modification steps shall be monitored by a combination of impedance spectroscopy and surface plasmon resonance spectroscopy, (SPR). Using electrochemical methods we will examine how efficient the ET takes place and what are the ET rate constants. In addition we are using the riboflavin binding protein dodecin, which binds flavins only when they are oxidized, in order to probe the flavin reduction. If it turns out that ds-DNA of 20 base pairs is too long for efficient ET, we will employ shorter hybrids of DNA and peptide nucleic acid (PNA). Two electrode architectures, which might serve in order to trace DNA mismatches, are shown below.
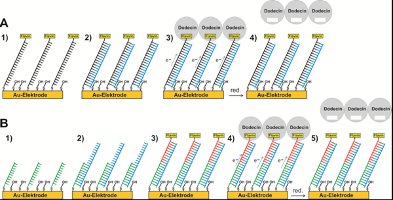
The sensor architecture shown in (A) consists of a flavin and thiol modified ss-DNA strand of 20 bases adsorbed to gold (1). In addition, a mercaptoalcohol has been adsorbed in order to displace non-specifically adsorbed DNA and to orient the ss DNA towards the solution in order to facilitate hybridization.
Besides the density of ss-DNA adsorbed to the surface, also the length of the mercaptoalcohol has to be optimized for efficient hybridization. After hybridization (2), the electrochemical reduction of the flavin by excess ET along the DNA base pairs should be possible. Further it should be possible to bind apododecin (3) and to release it by flavin reduction (4). Since ss DNA is highly flexible, the flavin could approach the surface and become reduced even without hybridization.
Dabei ist fraglich, ob bzw. wie beide Situationen (Flavinreduktion durch Annäherung an die Elektrodenoberfläche oder durch ET entlang der Basenpaare von ds-DNA nach vollständiger Hybridisierung) unterschieden werden können. Eine Alternative bietet der in (B) gezeigte Sensoraufbau, der aus einem adsorbierten PNA-Einzelstrang mit 10 Basen besteht (1). Erfolgt eine Hybridisierung mit der zu analysierenden DNA mit 20 Basen (2), wird im nächsten Schritt ein flavinmodifizierter PNA-Einzelstrang mit 10 Basen zugegeben. Nur wenn auch dieser eine komplementäre Basensequenz aufweist, ist ET möglich (3). In diesem Fall sollte es wiederum möglich sein, Apododecin anzubinden (4) und reduktiv freizusetzen. Die oben skizzierten Sensoren können noch dahingehend erweitert werden, dass nach Anbindung von Apododecin ein fluoreszenzfarbstoffmarkierter Flavinligand zugegeben und durch eine der weiteren Flavinbindungstaschen von Dodecin gebunden wird. In diesem Fall könnte die Anbindung und Freisetzung von Apododecin auch durch Oberflächenplasmonen-Fluoreszenzspektroskopie (SPFS) nachgewiesen werden. Sobald eine Konfiguration gefunden wird, in der effektiver ET durch DNA-Monolagen beobachtet werden kann und abgesichert ist, dass dieser ET entlang der Stapel von komplementären Basen-paaren abläuft, soll an der gezielten Aufspürung von einzelnen DNA-Fehlpaarungen gearbeitet werden.