inorganicmolecules
|
Inorganic Molecules (short summary of the treated topics)
|
| The lecture, test, and exercises “Inorganic Molecules” deal with the existence, formation, structure (electronic, molecular, crystal), and properties of inorganic molecular compounds and with some simple concepts/answers according to the questions what and why these molecules or compounds do exist with the respective structure and how to characterize them. 1. Lewis ConceptEach atom shares electrons with neighbouring atoms to achieve a total of two/eight/more valence electrons for Period 1/2/3… elements (closed-shell, noble-gas configuration, hypervalence/extended octet rule) in consideration of different resonance structures and/or resonance hybrids, and that all atoms want to have charges as low as possible.The knowledge of the Periodic Table (groups, periods) is sufficient (see frame Lewis). |
| 2. Valence Shell Electron Pair Repulsion Concept (VSEPR Concept)The corresponding concept is a good tool for predicting the shape or structure of molecules. It was established by Gillespie and Nyholm in 1957.For a molecule AXmEn (A= central atom, X= ligand atom, E= free electron pair), X and E tend to minimize repulsion, i.e. are arranged on the surface of a sphere around A forming simple polyhedra/geometric figures. Note that E (and double bonds) need more space than X. The polyhedra formed under inclusion of the free electron pairs are called Ψ-polyhedra (see frame VSEPR and http://www.shef.ac.uk/chemistry/vsepr ). |
|
3. Valence-bond theory (VB theory)
VB theory builds up the wavefunction of a molecule by superposition of the valence-wavefunction of the atoms involved in bond formation taking into account the constitution of the molecule and different Lewis structures. Hence VB theory is closer to the chemist´s idea of (localized) chemical bonding. To explain the structure/symmetry of molecules (e.g. CH4 with Td symmetry) hybridization of the atomic orbitals was introduced.
(see e.g. R. Hoffmann, S. Shaik, Ph. Hiberty, A Conversation on VB vs MO Theory: A Never-Ending Rivalty? Acc. Chem. Res. 2003, 36, 750-756.) |
|
4. Molecular orbital theory (MO theory)
MO theory approximates the wavefunction of a molecule by the linear combination of atomic orbitals (LCAO) using an (energy-) optimization procedure to reach self consistency. This results in delocalized molecular orbitals (MOs) which give either bonding or antibonding contributions to the molecule´s wavefunction. MO theory is easier to utilize in computer programs than VB theory.
For the construction of the MOs and some examples see frame MO. |
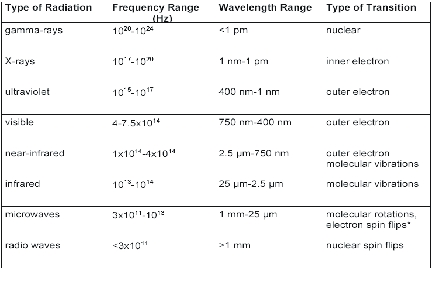
| 5. Shape/structure determination methodsUseful methods for determination of molecular/crystal shapes/structures and/or functional groups are mainly based on electromagnetic waves.Electromagnetic radiation consists of transversal waves with velocity c0 ≈ 3·108ms-1.The relevant characteristics are energy (frequency ν, wavelength λ, wavenumber ~ν ), intensity I, direction, phase φ, represented by the wave vector s0 (see frame Radiation). The structure determination methods are connected with the type (energy, frequency, and wavelength range) of radiation and the type of interaction/transition (see Table 5.1). Table 5.1 Types of electromagnetic radiation relevant for chemical spectroscopy
6. Exampels As examples for the above mentioned concepts/methods, the following elements/compounds/ anions have been treated/used: Carbon, phosphorus, sulfur, oxides, nitrogen oxides, nitrogen oxoions, phosphorus oxides, phoshorus oxoanions, sulfur oxoanions, selenites, sulfur halides, P-N, P-S, P-Se compounds (see http://www.webelements.com and Frame Examples). 7. Exercises For exercises see frame Exercises. |